
Restoring Nitrification in A Municipal Treatment System
Nitrogen is a key nutrient and essential for all living organisms for survival. It is a major part of amino acids, which are the building blocks of proteins. Proteins provide the structure for virtually all body tissues in humans and prevent plants from withering and dying. Additionally, proteins can function as enzymes and play a role in hormone production, affecting conservation and releasing of energy via metabolism (nitrogen also makes up a large part of chlorophyll needed for photosynthesis). Further, nitrogen is the key element in nucleic acids DNA—which carries genetic information—and RNA—which carries instructions from the DNA for genes—the most important of all biological molecules and needed for all living things.
About 78% of the atmosphere is made up of nitrogen but is inaccessible to organisms as a gas. The Nitrogen Cycle converts nitrogen into ammonia for organisms to use and incorporate into new proteins. Continue reading to learn how nitrogen goes from the atmosphere to living things through the Nitrogen Cycle and why it's important.
The Nitrogen Cycle
The Nitrogen Cycle is the cycle in which nitrogen moves through both living and non-living things. For nitrogen to move through the different parts of the cycle, it must change forms. The five stages of the Nitrogen Cycle describe the movement of nitrogen from place to place and the specific form it needs to take.
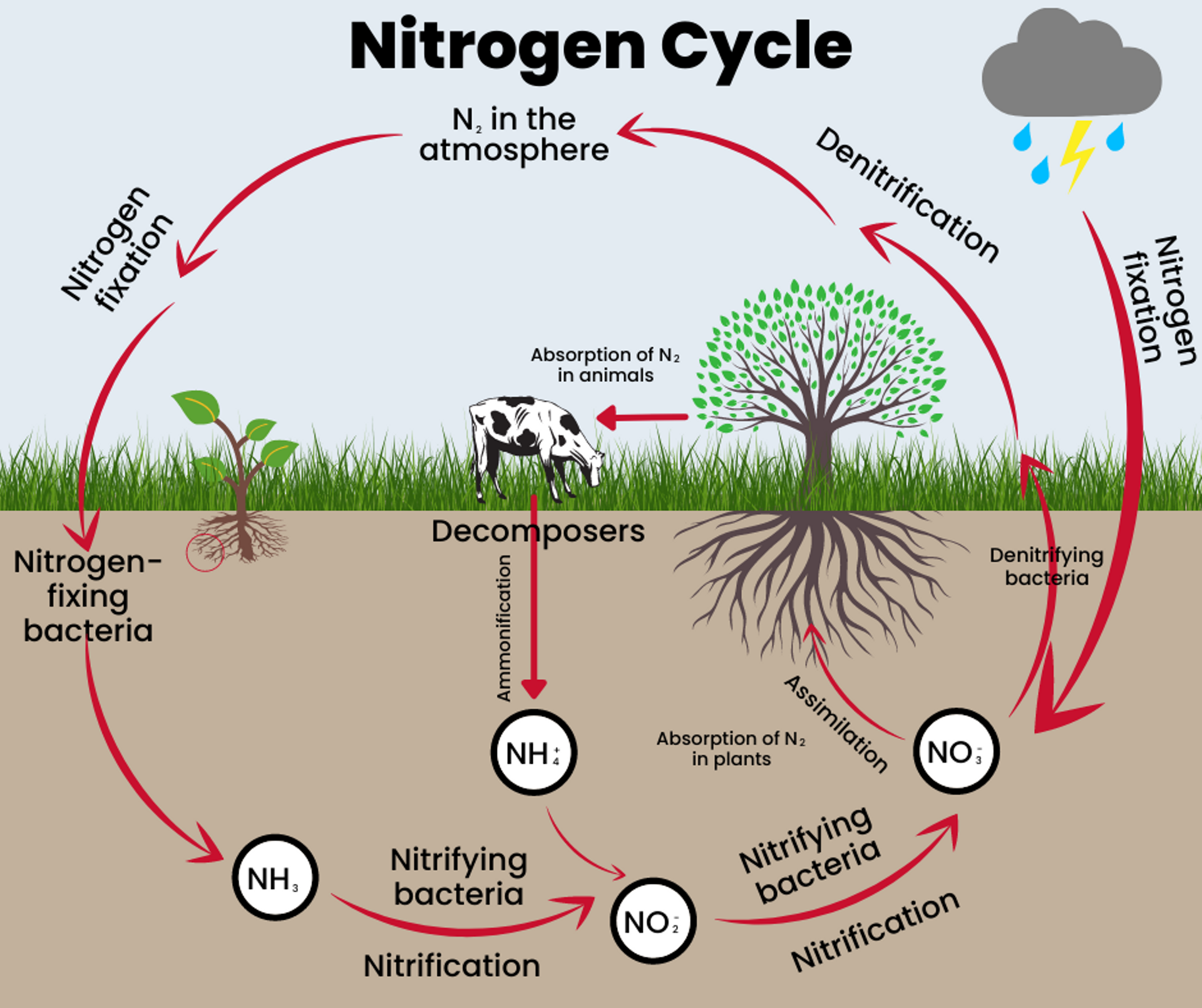
Stage 1:
Nitrogen fixation is when N2 (nitrogen in the atmosphere) is converted into a form plants can absorb through roots. Nitrogen is fixed when lightning provides the energy needed for N2 to react with oxygen, producing nitrogen oxide (NO) and nitrogen dioxide (NO2). These nitrogen forms enter the soil through rain or snow. Nitrogen fixation also naturally occurs in the soil by bacteria through photosynthesis.
Stage 2:
Mineralization takes place in soil when nitrogen moves from organic materials to an inorganic form of nitrogen that plants can use. Microbes act on animal manure and decomposing plant or animal material and begin to convert it from ammonia (NH3) to ammonium (NH4) to be used by plants that don’t get nitrogen through fixation.
Stage 3:
Nitrification also occurs in soils when the bacteria turn ammonia into compounds called nitrites (NO2-) and nitrates (NO3-). Nitrates are able to be used by organisms when they consume plants. Nitrites are not usable directly, but Nitrosomonas and Nitrobacter can cause the reaction to turn them into nitrates in the presence of oxygen.
Stage 4:
Immobilization controls and balances the amount of nitrogen in the soil by paralyzing the nitrogen in microorganisms. When microorganisms absorb ammonium and nitrates, it can cause nitrogen deficiency in plants, but immobilization prevents that from happening.
Stage 5:
Denitrification is when nitrogen returns to the air as nitrates are converted back into atmospheric nitrogen.
The Nitrification Process
Stage 3 of the Nitrogen Life Cycle is nitrification, when ammonia is converted to nitrites and then to nitrates by specialized bacteria. For this to actually happen, 2 steps need to take place:
- Ammonia oxidizes to a nitrite, which is carried out by Nitrosomonas plus hydrogen and water.
- The nitrite oxidizes to a nitrate, which is carried out by the bacterium Nitrobacter.
Nitrification is important in wastewater treatment systems because it is required in order to complete the treatment process. It removes nitrogen from the wastewater, which occurs as ammonium or bonded organic compounds. Wastewater treatment facilities that do not specifically remove nitrogen can lead to excess levels of nitrogen in groundwater. Excess nitrogen can harm bodies of water by increasing algae growth, which impacts food and habitats for aquatic life. It can also decrease oxygen in the water and affect water quality.
Temperature and the Nitrification Process
Nitrobacter and Nitrosomonas bacteria usually breakdown a significant amount of ammonia naturally in the right conditions. As temperature drops, however, these bacteria will see slower growth in the winter months, but discharge limits may not fluctuate enough to cover the higher levels of ammonia. The discharge limit is the maximum amount of pollutants that can be released, a regulation that aims to reduce nitrogen and protect water quality.
A fast drop in temperature or an incoming toxic event may not allow for bacteria to adjust quickly enough and results in higher untreated organic load. Temperature also impacts nitrifier regrowth during plant upsets. Nitrifiers, typically very slow growers, can take weeks to recover while bacteria can recover in days or hours. Cold temperatures can slow growth of nitrifiers from 12 to 24 hours, and bacteria can double in as little as 20 minutes. A dose of a nitrifier product will help by immediately increasing the number of microbes.
Case Study
A BOD municipal treatment plant with 2 million gallons of wastewater per day had shut down for 6 weeks for maintenance and a capital upgrade in late winter. 2 weeks after reopening, the system started losing its nitrification ability. Ammonia levels, which typically ran around 2-3 ppm, were now running at 18-21 ppm. In an effort to restore nitrification, the facility began importing 6,000 gallons of sludge per day from another facility. This solution brought the ammonia levels down to 16 ppm but was unable to drop it below that level.
The facility turned to EnviroZyme® for assistance by submitting a sample for microscopic analysis. The team determined it had a high level of encapsulation with extracellular polymeric substances (EPS) throughout the biomass, likely caused by loss of the biomass during the extended shutdown. Nitrifying bacteria, like Nitrosomonas, struggle to grow in a high EPS environment. It caused the loss of ammonia reduction capability.
In order to restore nitrification in the system, reduce EPS levels, and rebuild a healthy biomass, the team recommended the following:
- Supplement the system with Cold Weather Organics to quickly start building a healthy biomass, even in cold water temperatures
- Add Nitrifier to begin building the nitrifier population and restore ammonia reduction
To correctly dose the system, the treatment process was as follows:

- Day 1: Add 50 pounds of EZ Cold Weather Organics Control
- Day 2: Add an additional 25 pounds of EZ Cold Weather Organics Control and add 20 pounds of EZ Nitrification in liquid form
- Day 3: Add an additional 20 pounds of EZ Nitrification
Within 6 days of the first dose of product, the ammonia levels dropped.
- Day 2: 12.4 ppm
- Day 3: 10.6 ppm
- Day 5: 6.7 ppm
- The biomass was noted as healthy and active
- Day 6: 3.1 ppm
- Restored to baseline treatment levels
EnviroZyme’s bioaugmentation solutions quickly helped this wastewater treatment plant restore its nitrification, EPS levels, and biomass. The ammonia-reducing and nitrification-boosting products in our line of additives for system startups and upset recovery are specially formulated with a blend of nitrifying microorganisms to provide a consistent seed of both nitrifiers and denitrifiers for ammonia and nitrite conversion. Useful to accelerate the establishment of nitrification in newly commissioned or seasonally operated plants, as well as to assist in the maintenance of satisfactory nitrification in plants with a history of inconsistent performance, these products will help avoid fines and surcharges for non-compliant effluent ammonia.